39 fda approved statements about food components on food labels
Labeling and Label Approval | Food Safety and Inspection Service On October 13, 2021, the U.S. Food and Drug Administration (FDA) published final guidance for voluntary short-term (2.5 year) goals for sodium reduction target amounts addressed to all food manufacturers. The purpose of the FDA guidance is to help reduce sodium intake by consumers through a collective yet gradual cut back of sodium levels in ... Regulation of the U.S. Food Processing Sector — Food Law USDA pre-approves labels, FDA does not. For products regulated by FDA, the food business does its best to develop a label but it is only after it has begun to use the label that the business will learn whether FDA considers the label adequate. FSIS, as part of its continuous inspection, must approve a label before it can be used..
Fragrances in Cosmetics | FDA - U.S. Food and Drug Administration Feb 28, 2022 · Contact FDA Follow FDA on Facebook Follow FDA on Twitter View FDA videos on YouTube Subscribe to FDA RSS feeds FDA Homepage Contact Number 1-888-INFO-FDA (1-888-463-6332)

Fda approved statements about food components on food labels
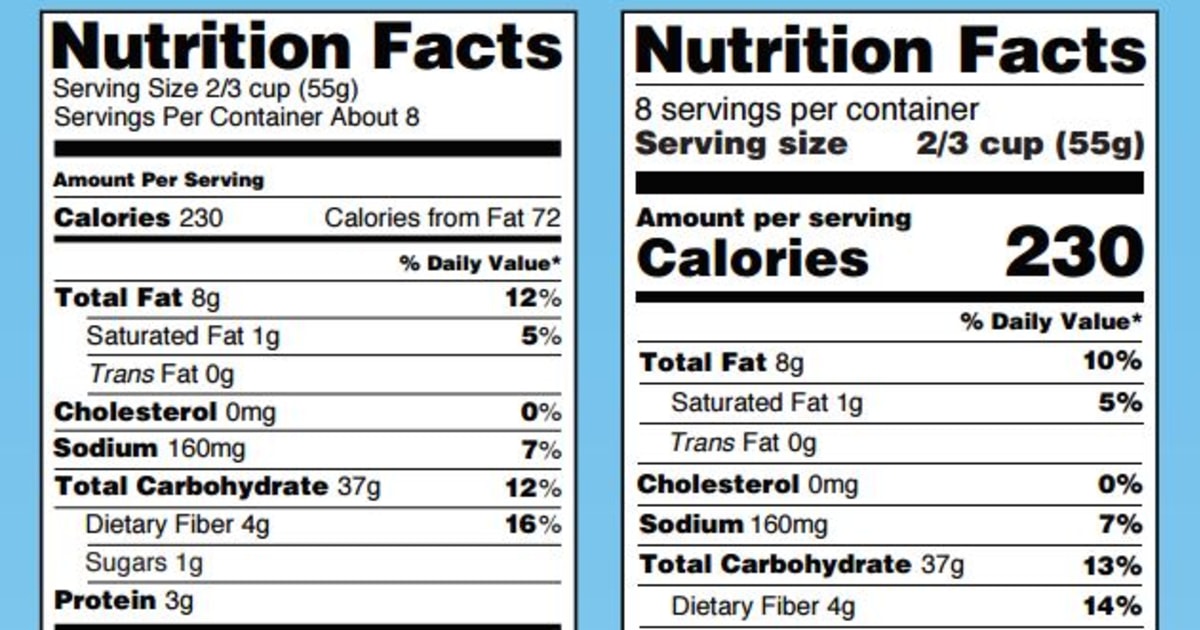
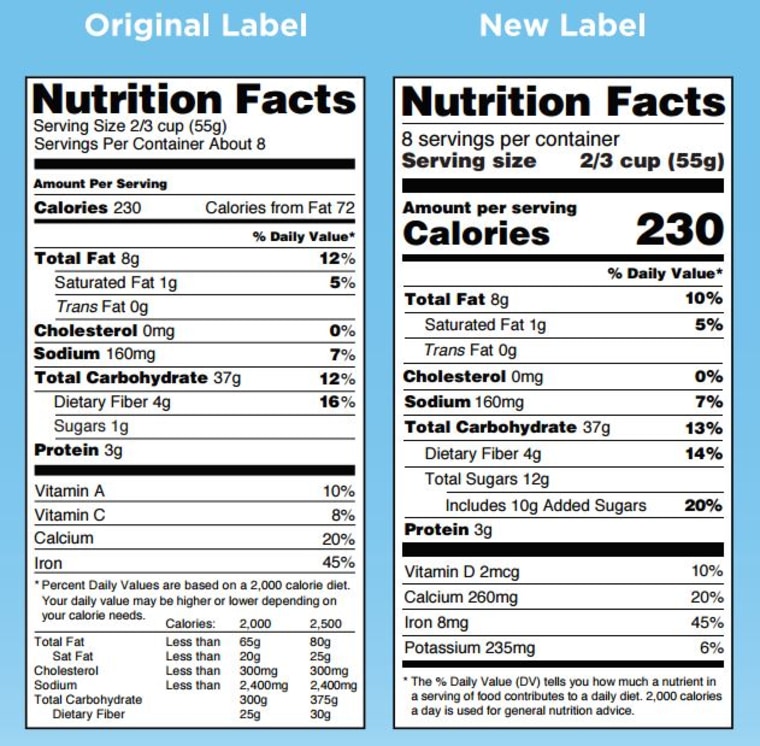
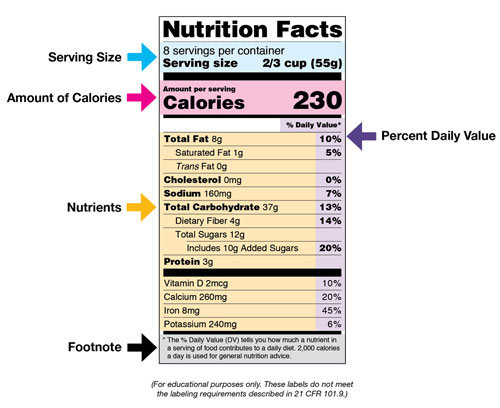
What is required on a food label? - USDA A meat and poultry label is required to contain 8 features. These are: the product name, inspection legend and est. number, handling statement, net weight statement, ingredients statement, address line, nutrition facts, and, safe handling instructions. These requirements are found in the Code of Federal Regulations (9CFR 317.2/381 Subpart N). eCFR :: 21 CFR Part 101 -- Food Labeling § 101.1 Principal display panel of package form food. The term principal display panel as it applies to food in package form and as used in this part, means the part of a label that is most likely to be displayed, presented, shown, or examined under customary conditions of display for retail sale. The principal display panel shall be large enough to accommodate all the mandatory label ... What's on the Nutrition Facts Label | UNL Food When the Food and Drug Administration (FDA) approved to update parts of the Nutrition Facts label, food manufacturers have been slowly changing their packaging to the new label. The original and newer labels appear different. Although the purpose of these labels has remained the same, understanding how they have changed can better help consumers make food choices that fit their needs.
Fda approved statements about food components on food labels. Food Labeling 101 - FDA Regulations Guide [2022] | Artwork Flow Food Labeling Requirements As Stated By The FDA, I. Principal Display Panel, 1. Brand Elements , 2. Statement of Identity, 3. Net Quantity, II. Information Panel, 1. Ingredient List, 2. Instructions to Use , 3. Manufacturer Name & Address, 4. Country of Origin, 5. Product Code, III. Nutrient Panel, 1. Nutrient Labeling, 2. Serving Sizes, IV. Food Ingredients & Packaging | FDA Irradiation of Food & Packaging, FDA provides regulatory and scientific information about irradiated food and packaging. Irradiation may be used to increase shelf-life and reduce harmful bacteria... FDA Labeling Requirements for Food - What You Need to Know - Thomasnet Food labeling is required by law for most prepared foods, such as loaves of bread, cereals, canned and frozen foods, desserts, snacks, drinks, etc. Nutrition labeling for raw produce, i.e., fruits and vegetables, and for fish, is voluntary. The FDA refers to these products as "conventional" foods. Label Claims for Conventional Foods and Dietary Supplements A Food Labeling Guide - Appendix C: Health Claims. A "health claim" by definition has two essential components: (1) a substance (whether a food, food component, or dietary ingredient) and (2) a ...
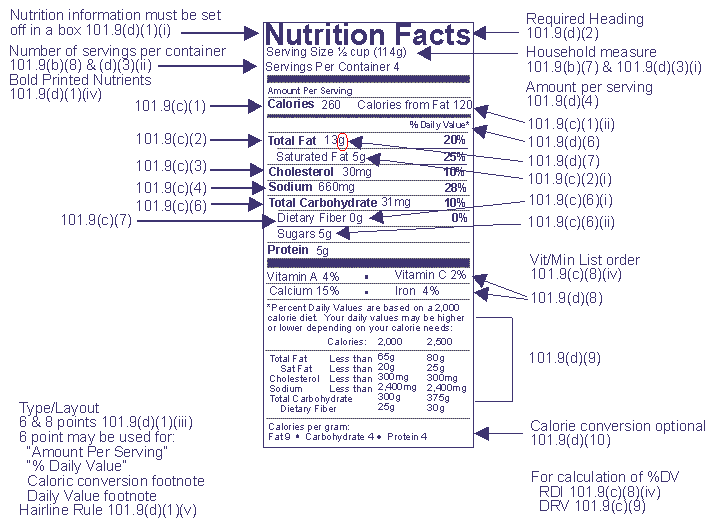
Guidance for Industry: Food Labeling Guide | FDA The Federal Food, Drug, and Cosmetic Act (FD&C Act) and the Fair Packaging and Labeling Act are the Federal laws governing food products under FDA's jurisdiction. The FDA receives many questions... PDF SUMMARY OF 5 REQUIRED FOOD LABEL COMPONENTS Label Layout Instructions ... FDA regulations require components of every retail food package with positioning and minimum type size as outlined below. The sidebar picture shows a sample representation of a Principal Display Panel (PDP) and an Information Panel (IP). The PDP is the front of the package; the IP is the panel immediately to the right of the PDP. Positioning and... PDF Food Labeling Guide - Food and Drug Administration Food Labeling Guide, Additionalcopies are available from: Office of Nutrition, Labeling, and Dietary Supplements HFS-800 Center for Food Safety and Applied Nutrition Food and Drug Administration... FDA Regulation of Cannabis and Cannabis-Derived Products ... In addition, under 21 CFR 530.20(b)(2), if scientific information on the human food safety aspect of the use of the approved human drug in food-producing animals is not available, the veterinarian ...
General Food Labeling Requirements - California FDA has approved various health claims based on extensive scientific evidence and defined conditions under which the claims can be used (e.g., sodium and hypertension, calcium and osteoporosis). For more information, refer to 21 CFR § 101.72 101.83, the . Label Claims . or the . Guidance for Industry: A Food Labeling Guide at the FDA website. Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and... Food Packaging Claims | American Heart Association There are three categories of claims defined by statute and/or FDA regulations that can be used on food and dietary supplement labels: health claims, nutrient content claims, and; structure/function claims. A "health claim" by definition has two essential components: A substance (whether a food, food component, or dietary ingredient) and PDF A Guide to Federal Food Labeling Requirements for Meat and Poultry Products The food label is important to food companies and consumers alike. A company's most direct (and sometimes only) way to communicate with the consumer is via the food label. For consumers, the food label contains a wealth of information, which allows for informed purchase decisions. The U.S.
FDA Food Product Labeling & Packaging Requirements - ESHA The Food Allergen Labeling and Consumer Protection Act of 2004 (FALCPA) mandates that packaged food items must declare, in plain language, the presence of any major food allergens (Milk, Egg, Fish, Crustacean shellfish, Tree nuts, Wheat, Peanuts, Soybeans, Sesame) on the product packaging.
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration The information on this page is current as of Jul 20, 2022. For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 184.1400 Lecithin. (a) Commercial lecithin is a naturally occurring mixture of the phosphatides of choline, ethanolamine, and inositol, with smaller amounts of other lipids ...
FDA Compliant, Food Grade and Food Safe | ISM - Industrial Spec FDA compliant is a shorthand way of talking about materials that are safe for direct food contact. These materials are also called food contact substances (FCS). An FCS is any material that comes into contact with or is used for manufacturing, packing, packaging, transporting or holding food. Both the plastics and the pigments used in making ...
The New Nutrition Facts Label | FDA - U.S. Food and Drug Administration The U.S. Food and Drug Administration (FDA) has updated the Nutrition Facts label on packaged foods and drinks. FDA is requiring changes to the Nutrition Facts label based on updated scientific...
Simethicone | C6H18O4Si3 - PubChem The Approved Drug Products with Therapeutic Equivalence Evaluations identifies currently marketed over-the-counter drug products, including simethicone, approved on the basis of safety and effectiveness by FDA under sections 505 of the Federal Food, Drug, and Cosmetic Act.
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Sec. 175.105 Adhesives. (a) Adhesives may be safely used as components of articles intended for use in packaging, transporting, or holding food in accordance with the following prescribed conditions: (1) The adhesive is prepared from one or more of the optional substances named in paragraph (c) of this section, subject to any prescribed ...
Creating an Ingredients List on a Nutrition Label: A Guide to FDA ... Simply search from our extensive ingredient library of over 18,000 items, to find the ingredients in your recipe (e.g. 1 cup of flour, 2 oz of butter), and LabelCalc will automatically calculate the nutrition analysis, your product-specific serving size, servings per container, and generate an ingredient statement with any relevant allergy warnings.
Principles of Nutrition Ch. 1-4 Flashcards | Quizlet Minerals and water, do not contain carbon, Organic nutrients, carbohydrate, proteins, fats, and vitamins, contain carbon, energy yielding nutrients, carbohydrates, proteins, and fats, placebo effect, "mind-body" effect, taking anything believed to be beneficial that may hasten recovery, scientific method,
Food Labels Guide & Examples | How to Read Nutrition Labels - Video ... The inclusion of food claims on a label about this connection has been approved by the FDA. Whole grains reduce the risk of type 2 diabetes. Producers have been allowed to mark food claims about ...
Packaging, Labeling, Transporting, Storing — Food Law FDA does not pre-approve labels; FDA may offer suggestions if the processor inquires; FDA will enforce law after the label is put in use. No pre-approval of label is required by FDA for products under its jurisdiction. It is the responsibility of the manufacturer or importer of a food to comply with current food labeling regulations."
FDA: Foods Must Contain What Label Says | FDA - U.S. Food and Drug ... After conducting its own analyses, FDA found that some of the samples contained undeclared ingredients, including artificial colors, sweeteners and less expensive fruit juices, such as black...
Ingredients Guidance | Food Safety and Inspection Service This document is intended to provide guidance to interested parties who wish to use new food ingredients and sources of radiation or to make new use of approved food ingredients and sources of radiation in the manufacture of meat and poultry products. Memorandum of Understanding,
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration All approved material is available for inspection at the Office of Nutrition and Food Labeling (HFS-800), Center for Food Safety and Applied Nutrition, Food and Drug Administration, 5001 Campus Dr., College Park, MD 20740, 240-402-2404 and is available from the sources indicated below.
Nutrition Chapter 2 Flashcards | Quizlet These are FDA-approved statements about food components on food labels. balance, This means eating some food from each food group. nutrient density, This is an indicator of which food provides the most nutrients for the least kcalories. adequacy, This is the situation when enough calories and nutrients are provided in the diet. moderation,
eCFR :: 21 CFR Part 312 -- Investigational New Drug Application (a) This part contains procedures and requirements governing the use of investigational new drugs, including procedures and requirements for the submission to, and review by, the Food and Drug Administration of investigational new drug applications (IND's). An investigational new drug for which an IND is in effect in accordance with this part is exempt from the premarketing approval ...
FSIS Compliance Guideline for Label Approval | Food Safety and ... Guideline ID FSIS-GD-2017-0011. Issue Date July 2020. Full Guideline. FSIS Compliance Guidance for Label Approval. Replaces: August 2017 version. This guidance document provides official establishments information about the types of labels that must be submitted to LPDS for approval. Included are specific examples of special statements and ...
What's on the Nutrition Facts Label | UNL Food When the Food and Drug Administration (FDA) approved to update parts of the Nutrition Facts label, food manufacturers have been slowly changing their packaging to the new label. The original and newer labels appear different. Although the purpose of these labels has remained the same, understanding how they have changed can better help consumers make food choices that fit their needs.
eCFR :: 21 CFR Part 101 -- Food Labeling § 101.1 Principal display panel of package form food. The term principal display panel as it applies to food in package form and as used in this part, means the part of a label that is most likely to be displayed, presented, shown, or examined under customary conditions of display for retail sale. The principal display panel shall be large enough to accommodate all the mandatory label ...
What is required on a food label? - USDA A meat and poultry label is required to contain 8 features. These are: the product name, inspection legend and est. number, handling statement, net weight statement, ingredients statement, address line, nutrition facts, and, safe handling instructions. These requirements are found in the Code of Federal Regulations (9CFR 317.2/381 Subpart N).











/Food-label-Envision-575f13f25f9b58f22ee9a2dc.jpg)











Post a Comment for "39 fda approved statements about food components on food labels"